TDP-43 IMR Reagent
“MagQu” TDP-43 IMR Reagentt is designed for quantitative measurement of Tau protein concentration in human plasma by immunomagnetic reduction (IMR) assay. The reagent can be used with MagQu’s Magnetic Immunoassay Analyzer XacPro-S system.
This assay enables early-stage neurological disease research with ultra-high sensitivity and low interference.
Features
Quantifying TDP-43 in the sample easily, rapidly, and accurately
Magnetic Nanoparticle
Dextran layer
For ALS, FTLD and Alzheimer’s disease research and for in-vitro diagnosis use
Specifications
Sample type: Human Plasma
Sample volume: 60 μl
Assay time: 5 hours (36 channels in XacPro-S)
Use application: In vitro diagnostic
Detection methods: ImmunoMagnetic Reduction (by analyzer XacPro-S with magnetic reagents)
Sensitivity
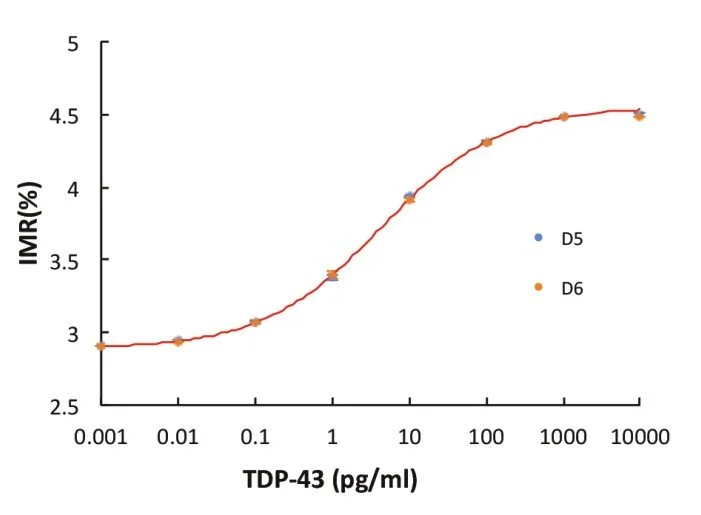
Detection Range: 0.001 - 100 pg/ml
Low detection limit: 0.68 fg/ml
IMR standard curve of TDP-43
Description
Intended Use
“MagQu” TDP-43 IMR Reagent is used to quantitatively measure TDP-43 protein in human fluid specimen, such as plasma, serum or CSF.
Use “MagQu” TDP-43 IMR Reagent only with the XacPro-S System (MagQu Co., Ltd.).
Introduction
The TAR DNA binding protein of 43 kDa (TDP-43) is a ubiquitously expressed nuclear protein with roles in transcription and splicing regulation. A hyper-phosphorylated, ubiquitinated and cleaved form of TDP-43, known as pathologic TDP-43 is the major disease protein in ubiquitin-positive, tau and α-synuclein-negative frontotemporal dementia (FTLD-TDP, previously referred to as FTLD-U) and in amyotrophic lateral sclerosis (ALS). Evidences indicates that TDP-43 can be detected in human plasma and CSF and levels are reportedly elevated in cases of ALS, FTLD and Alzheimer’s disease. 1,2
Principles of Test
“MagQu” TDP-43 IMR Reagent is designed for rapid quantifying TDP-43 by ImmunoMagnetic Reduction (IMR). We conjugate antibody on the surface of around 50 nm-in-diameter Fe3O4 magnetic particles. When the antibodies on the surface bind with TDP-43, the magnetic particles form clusters. Therefore, the ac susceptibility (Xac) of magnetic particles would be reduced in the adding ac magnetic field. By measuring the reduction of Xac, TDP-43 can be easily, rapidly and accurately quantified.3
Reagent Properties
Precision
The TDP-43 samples were measured in duplicate,
twice per day over 20 days.
Two different TDP-43 concentrations were used for
the tests. The standard deviations of repeatability
and within-lab for various TDP-43 concentrations
ware obtained.
Interference (Specificity)
Plasma can contain interfering substances such
as hemoglobin, bilirubin, or intra lipid because of
common diseases, such as hemolysis, jaundice or
hypertriglyceridemia. Other bio-substances that
exist naturally in plasma, such as uric acid, rheu-
matoid factor, or albumin, are also interfering
substances. Other interfering substances include
drugs or chemicals in medicine that is used to
treat inflammatory diseases, viral and bacterial
infections, cancers and cardiovascular disease.
The level of TDP-43 in each of these pools was
then determined and normalized to the level
without the respective substances.
Expected Value
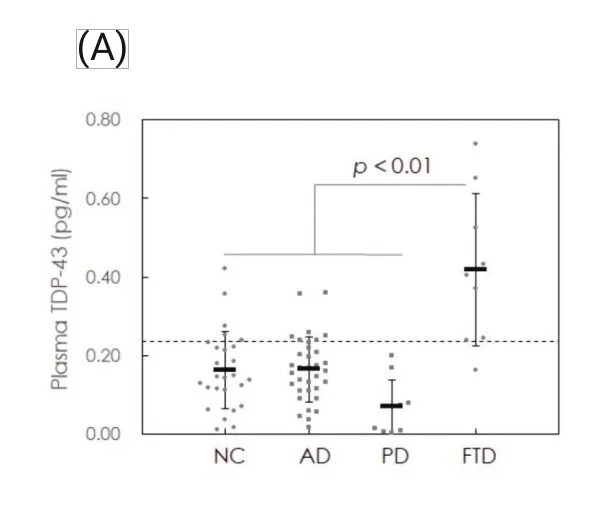
Plasma samples from normal controls (NC, n = 27) and from patients with frontotem- poral dementia (FTD, n = 9), Alzheimer’s disease (AD, n = 34) and Parkinson’s disease (PD, n = 10) were collected for TDP-43 assays using the IMR TDP-43 reagent.
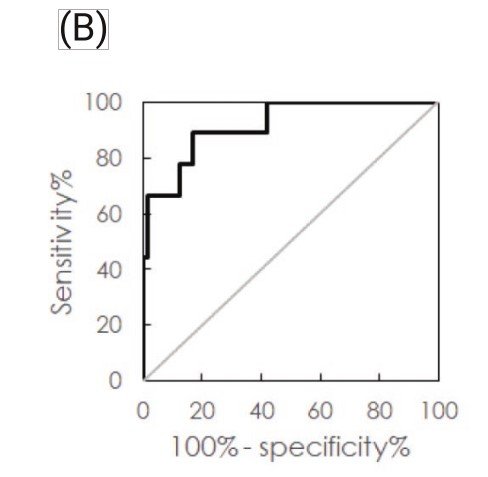
Through analysis of the ROC curve, the cut-off value to discriminate FTD from the other groups, including NC, AD and PD, was found to be 0.237 pg/ml. The clinical sensi- tivity and specificity were 88.9% and 83.1%, respectively. The area under the curve was 0.917.
(A) Measured plasma TDP-43 concentrations in normal controls (NC) and patients with Alzheimer’s disease (AD), Parkinson’s disease (PD) or frontotemporal dementia (FTD), (B) ROC curve for discriminating FTD from other groups using plasma TDP-43 concentration.
Application References:
Yang SY, Liu HC, Lin CY, Chiu MJ, Chen TF, Lin CH, Chen HH. Development of Assaying Plasma TDP-43 Utilizing Immunomagnetic Reduction. J Neurol Disord, 2020; 8(7).