Phospho-α-Synuclein IMR Reagent
“MagQu” Phospho-α-Synuclein IMR Reagent is designed for quantitative measurement of Tau protein concentration in human plasma by immunomagnetic reduction (IMR) assay. The reagent can be used with MagQu’s Magnetic Immunoassay Analyzer XacPro-S system.
This assay enables early-stage neurological disease research with ultra-high sensitivity and low interference.
Features
Quantifying Phosphorylated α-synuclein(p-ASC129) in the sample easily, rapidly, and accurately
Magnetic Nanoparticle
Dextran layer
For Parkinson’s disease (PD) research and for in-vitro diagnosis use
Specifications
Sample type: Human Plasma
Sample volume: 60 μl
Assay time: 5 hours (36 channels in XacPro-S)
Use application: In vitro diagnostic
Detection methods: ImmunoMagnetic Reduction (by analyzer XacPro-S with magnetic reagents)
Sensitivity
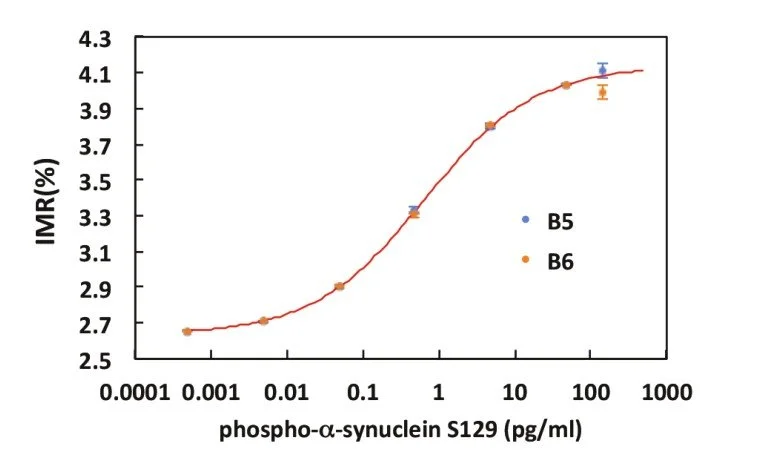
Detection Range: 0.00048 - 0.4826 pg/ml
Low detection limit: 0.072 fg/ml
IMR standard curve of phospho -α-synuclein (Serine 129)
Description
Intended Use
“MagQu” Phospho-α-Synuclein IMR Reagent is used to quantitatively measure phosphorylated α-synuclein (p-ASC129) in human fluid specimen, such as plasma, serum or CSF. Use “MagQu” Phospho-α-Synuclein IMR Reagent only with the XacPro-S System (MagQu Co., Ltd.).
Introduction
Parkinson’s disease (PD) is characterized by the intraneuronal α-synuclein inclusions called Lewy bodies. Increase of phosphorylation of α-synuclein in Serine 129 (p-ASC129) has been correlated with the aggregation, toxicity, protein interaction and turnover of α-synuclein. Thus, p-ASC129 can indicate the pathogenesis of PD.1,2
Principles of Test
“MagQu” Phospho-α-synuclein IMR Reagent is designed for rapid quantifying phosphorylated α-synuclein (p-ASC129) by ImmunoMagnetic Reduction (IMR). We conjugate antibody on the surface of around 50 nm-in-diameter Fe3O4 magnetic particles. When the antibodies on the surface bind with phosphorylated α-synuclein (p-ASC129), the magnetic particles form clusters. Therefore, the ac susceptibility (Xac) of magnetic particles would be reduced in the adding ac magnetic field. By measuring the reduction of Xac, phosphorylated α-synuclein (p-ASC129) can be easily, rapidly and accurately quantified.3
Reagent Properties
Precision
The phosphorylated α-synuclein (S129) samples
were measured in duplicate, twice per day over 20
days. Two different phosphorylated α-synuclein
(S129) concentrations were used for the tests.
The standard deviations of repeatability and with-
in-lab for various phosphorylated α-synuclein
(S129) concentrations ware obtained.
Interference (Specificity)
Plasma can contain interfering substances such as
hemoglobin, bilirubin, or intra lipid because of common
diseases, such as hemolysis, jaundice or hypertriglyceri-
demia. Other bio-substances that exist naturally in
plasma, such as uric acid, rheumatoid factor, or albumin,
are also interfering substances. Other interfering
substances include drugs or chemicals in medicine that
is used to treat inflammatory diseases, viral and bacte-
rial infections, cancers and cardiovascular disease. The
level of phosphorylated α-synuclein (S129) in each of
these pools was then determined and normalized to the
level without the respective substances.
Expected Value
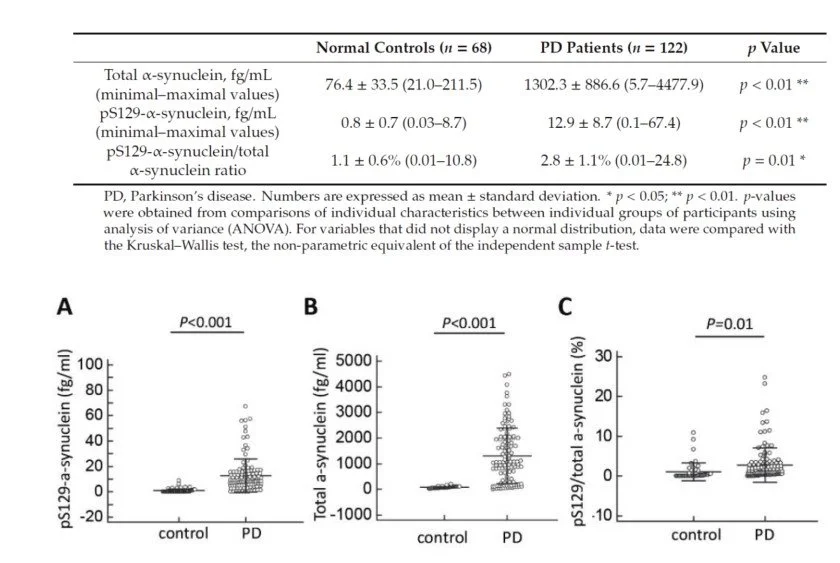
A total of 190 study participants, including 122 Parkin- son’s disease (PD) patients and 68 normal controls, were enrolled.
Fig 1. Plasma total and phosphorylated Ser129-synuclein levels for all participants in the study. (A) The plasma levels of pS129-synu- clein, (B) total-synuclein level and (C) the pS129/total-synuclein ratio were significantly increased in patients with Parkinson’s disease (PD) compared to normal controls.
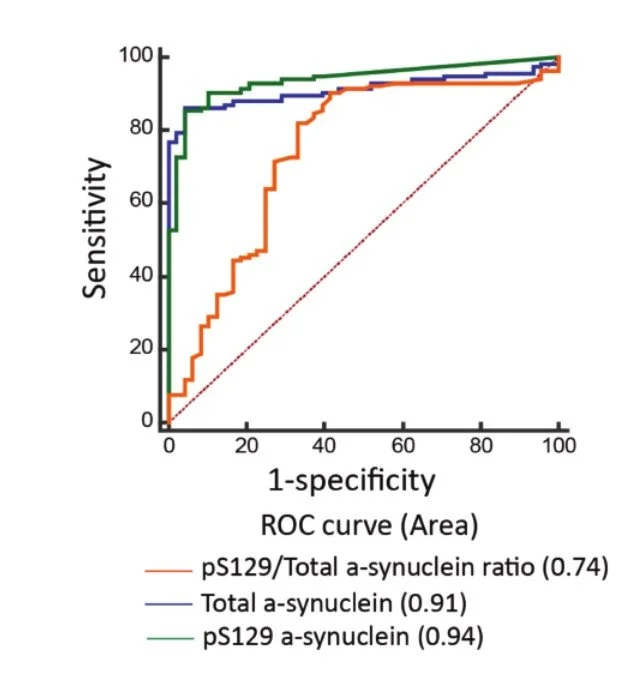
Fig 2. Receiver operating characteristic curves for predicting disease in participants. The accuracy of predicting Parkinson’s disease using the pS129/total-synuclein ratio (area under curve (AUC) = 0.63), total -synuclein level (AUC = 0.91), and pS129--synu- clein (AUC = 0.94).
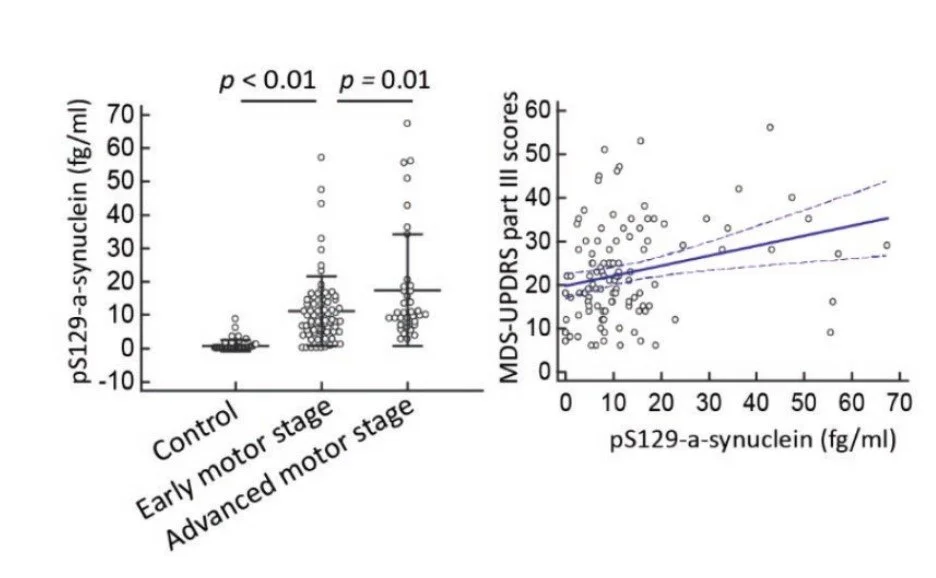
Fig 3. Plasma pS129-synuclein levels in Parkinson’s disease (PD) patients with varying disease severity. (A) The plasma pS129-synu- clein level was markedly increased in PD patients with more severe motor disability as assessed by Hoehn–Yahr stage (p < 0.01) and (B) correlated with motor symptom severity as measured by MDS-UPDRS part III scores (r = 0.27 (95% CI: 0.09–0.43), p = 0.004).
Application References:
Lin CH, Liu HC, Yang SY, Yang KC, Wu CC, Chiu MJ. Plasma pS129-α-Synuclein Is a Surrogate Biofluid Marker of Motor Severity and Progression in Parkinson's Disease. J Clin Med. 2019 Oct 3;8(10):1601.