BDNF IMR Reagent
“MagQu” BDNF IMR Reagent is designed for quantitative measurement of Tau protein concentration in human plasma by immunomagnetic reduction (IMR) assay. The reagent can be used with MagQu’s Magnetic Immunoassay Analyzer XacPro-S system.
This assay enables early-stage neurological disease research with ultra-high sensitivity and low interference.
Features
Quantifying BDNF in the sample easily, rapidly, and accurately
Magnetic Nanoparticle
Dextran layer
For depression, dementia and Alzheimer's disease research and for in-vitro diagnosis use
Specifications
Sample type: Human Plasma
Sample volume: 60 μl
Assay time: 5 hours (36 channels in XacPro-S)
Use application: In vitro diagnostic
Detection methods: ImmunoMagnetic Reduction (by analyzer XacPro-S with magnetic reagents)
Sensitivity
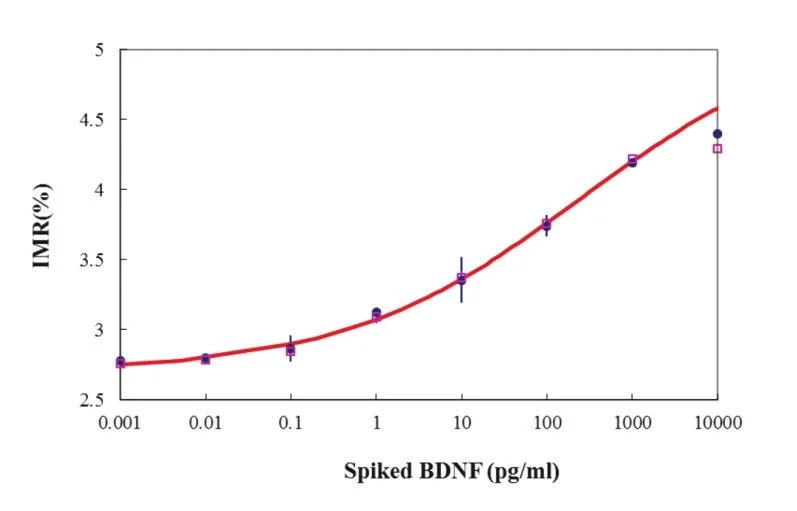
Detection Range: 10 - 1000 pg/ml
Low detection limit: 3 fg/ml
IMR standard curve of Tau Protein
Description
Intended Use
“MagQu” BDNF IMR Reagent is used to quantitatively measure brain-derived neurotrophic factor(BDNF) in human fluid specimen, such as plasma, serum or CSF.
Use “MagQu” BDNF IMR Reagent only with the XacPro-S System (MagQu Co., Ltd.).
Introduction
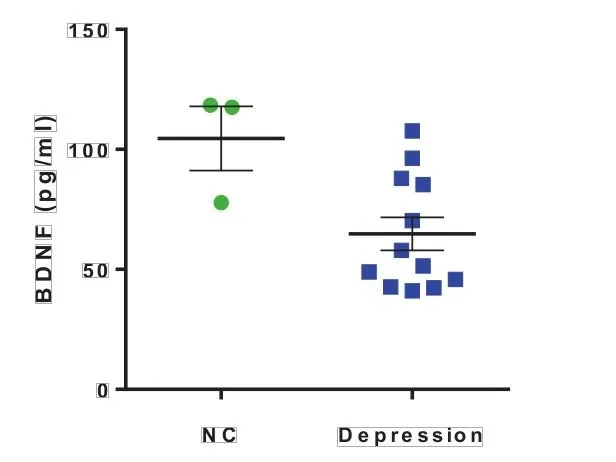
Brain-derived neurotrophic factor (BDNF) is a family of neurotrophic proteins that promote neuronal survival, normal function and development and plays an important role in the regulation of cognition and memory. BDNF has been previously found to be associated with depression and studies have found that depressed patients have a significant decrease in BDNF expression. In recent years, BDNF has also been found to be associated with dementia. Previous studies have shown that high levels of BDNF expression in the peripheral system can protect older adults against Alzheimer's disease. Therefore, low BDNF expression is considered to be a precursor to Alzheimer's disease. The reason for this low level of BDNF expression may be that when the neurons of dementia patients start to be damaged, the entire brain environment starts to deteriorate, and the nerves, glial cells and endothelial cells that secrete BDNF are also damaged, thus reducing the amount of secretion. This suggests that BDNF is a novel predictor of depression, dementia and Alzheimer's disease in healthy adults, as well as a biomarker of dementia risk and prognosis..1,2,3
Principles of Test
“MagQu” BDNF IMR Reagent is designed for rapid quantifying BDNF by ImmunoMagnetic Reduction (IMR). We conjugate antibody on the surface of around 50 nm-in-diameter Fe3O4 magnetic particles. When the antibodies on the surface bind with BDNF, the magnetic particles form clusters. Therefore, the ac susceptibility (Xac) of magnetic particles would be reduced in the adding ac magnetic field. By measuring the reduction of Xac, BDNF can be easily, rapidly and accurately quantified.4
Reagent Properties
Precision
The BDNF samples were measured in duplicate,
twice per day over 20 days. Two different BDNF
concentrations were used for the tests. The
standard deviations of repeatability and within-lab
for various BDNF concentrations ware obtained.
Interference (Specificity)
Plasma can contain interfering substances such
as hemoglobin, bilirubin, or intra lipid because of
common diseases, such as hemolysis, jaundice
or hypertriglyceridemia. Other bio-substances
that exist naturally in plasma, such as uric acid,
rheumatoid factor, or albumin, are also interfer-
ing substances.
Other interfering substances include drugs or
chemicals in medicine that is used to treat
inflammatory diseases, viral and bacterial infec-
tions, cancers and cardiovascular disease. The
level of BDNF in each of these pools was then
determined and normalized to the level without
the respective substances.
Expected Value
Plasma samples from normal controls (NC, n = 3) and from patients with depression (n = 12) were collected for BDNF assays using the BDNF reagent.
Fig. 1 Measured concentrations of plasma BDNF